New FICR Facility Policies in place due to Covid-19 Restrictions
Facility access will be dictated by university/hospital mandated building closures for daily disinfection/cleaning.
Mandatory PPE, social distancing and new disinfection policies will be in effect throughout the facility.
Instrument Training will be suspended until social distancing guidelines are relaxed.
NO WALK-UP INSTRUMENT USE WILL BE PERMITTED; all use must be reserved in iLab prior to the appointment time so it can be approved by core staff.
Existing users will reserve instruments through iLab as usual, but all reservations will require core approval, so advance planning is required.
If you are in need of assisted sorting or analysis, please contact Viraga.haridas@childrens.harvard.edu
Welcome to the Flow and Imaging Cytometry Resource (FICR) at Boston Children's Hospital.
FICR provides a platform and instrumentation for researchers interested in using flow and imaging cytometry, as well as molecular imaging for their biological and biomedical research questions.
FICR provides scientists with access to a number of critical technologies including Flow Cytometry and Cell Sorting, Imaging Cytometry and In Vivo Molecular Imaging.
Viraga Haridas, Ph.D; Core Director, Flow and Imaging Cytometry Resource
James V. Falvo, Ph.D.; Scientific Administrative Director, Program in Cellular and Molecular Medicine
| Hours | Locations |
|
Open All Hours
|
Armenise Building (200 Longwood Ave): AR-231 and AR-239 Center for Life Sciences (3 Blackfan Cr): CL-03034 and CL-3137 New Research Building: Basement NRB 0044 |
Users will be billed for the time they reserve on the iLab calendar OR the actual instrument time used, whichever is greater.
There is a 24-hour cancellation policy. Users that do not cancel or show up for their reservations will be billed for the time reserved, unless they contact FICR staff in advance of their scheduled appointment time to cancel or modify the reservation. All scheduling changes requested within 24 hours of the reserved time will be made at the discretion of FICR staff.
Users that reserve instruments in the facility must abide by the policies set forth by the facility.
FACS Cell Sorters and Analyzers (Self operated after training)
SONY SH-800Z: 4-Laser, 6-Color . Warren Alpert building (WA-138) and Center for Life Sciences Building (CL-3117)

Lasers: 488 nm, 405 nm, 561 nm and 638 nm
Filters:
450/50: PacBlue/DAPI (FL1)
525/50: FITC/GFP (FL2)
585/15: PE (FL3)
665/30: APC (FL4)
720/60: AF700/PCP-Cy5.5 (FL5)
785/60: PE-Cy7/APC-Cy7 (FL6)
FACSARIA II SORP: 5-Laser, 16-Color. Armenise building (AR-239)
488 nm 406 nm 592 nm 640 nm 355 nm
530/30: FITC 450/50: PacBlue 630/20: mCherry 660/20: APC 383/17: BUV395
575/25: PE 530/30: AmCyan 670/20: DsRed (APC) 720/40: AF700 530/30: Indo-1
605/12: PE-Cy5 605/12: QDot605 780/60: APC-Cy7
670/30: PCP-Cy5.5 705/70: QDot705
780/60: PE-Cy7
Cytek Aurora: 4-Laser, 48-Florescence Parameters, 3 Scatter Channels. Armenise building (AR-239)

Lasers: 405 nm, 488 nm, 561 nm and 640 nm
Runs 5ml tubes, Automated Sample Loader (ASL) which supports a variety of 96-well plates.
405nm 488nm 561nm 640nm
Channel Bandpass Wavelength Range Channel Bandpass Wavelength Range Channel Bandpass Wavelength Range Channel Bandpass Wavelength Range
V1 428/15 420-435 B1 508/20 498-518 YG1 577/20 567-587 R1 661/17 653-670
V2 443/15 436-451 B2 525/17 516-533 YG2 598/20 588-608 R2 679/18 670-688
V3 458/15 451-466 B3 542/17 533-550 YG3 615/20 605-625 R3 697/19 688-707
V4 473/15 466-481 B4 581/19 571-590 YG4 661/17 653-670 R4 717/20 707-727
V5 508/20 498-518 B5 598/20 588-608 YG5 679/18 670-688 R5 738/21 728-749
V6 525/17 516-533 B6 615/20 605-625 YG6 697/19 688-707 R6 760/23 749-772
V7 542/17 533-550 B7 661/17 653-670 YG7 720/29 706-735 R7 783/23 7 72-795
V8 581/19 571-590 B8 679/18 670-688 YG8 750/30 735-765 R8 812/34 795-829
V9 598/20 588-608 B9 697/19 688-707 YG9 780/30 765-795
V10 615/20 605-625 B10 717/20 707-727 YG10 812/34 795-829
V11 664/27 651-678 B11 738/21 728-749
V12 692/28 678-706 B12 760/23 749-772
V13 720/29 706-735 B13 783/23 772-795
V14 750/30 735-765 B14 812/34 795-829
V15 780/30 765-795
V16 812/34 795-829
FACSCanto II-AR: 3-Laser, 8-Color. Armenise building (AR-239)
488 nm 405 nm 640 nm
530/30: FITC 450/50: PacBlue 660/20: APC
562/42: PE 510/50: AmCyan 780/60: APC-Cy7
670 LP: PCP/PCP-Cy5.5
780/60: PE-Cy7
FACSCanto II-CLSB: 3-Laser, 8-Color. 3-Blackfan Circle (CLSB 3rd Floor, CL-03034) With Plate loader
488 nm 405 nm 640 nm
530/30: FITC 450/50: PacBlue 660/20: APC
562/42: PE 510/50: AmCyan 780/60: APC-Cy7
670 LP: PCP/PCP-Cy5.5
780/60: PE-Cy7
Hybrid "In Flow" Microscope - ImageStreamX Mark II (self-operated after training).
Armenise building (AR-231)


|
Imaging flow Cytometry with Hybrid "In Flow" Microscope |
Optical Configuration and Parameters |
Applications |
|
Quantitative imaging flow cytometry (IFC) allows to analyze cells, cellular clusters,aggregates, and extracellular vesicles with sensitivity higher than conventional flow cytometer
|
|
|
Molecular Imaging In Vivo/ Pre-clinical in vivo imaging (self-operated after training).
Location: New Research Building, Basement.

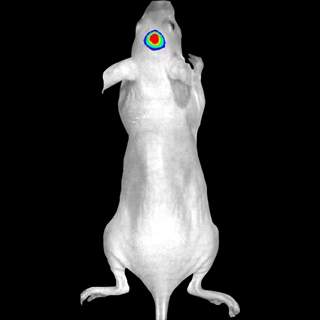
|
Advanced optical imaging |
Optical Configuration |
Applications |
|
The fluorescent and bioluminescent reporters |
28 efficiency filters spanning 430-850 nm; CCD camera: 13.5 micron pixels, 2048x2048 |
Non-invasive longitudinal monitoring of disease progression, cell trafficking and gene expression patterns in living animals |
|
|
|
| Name | Role | Phone | Location | |
|---|---|---|---|---|
| Viraga Haridas |
Core Director
|
339-970-1931
|
viraga.haridas@childrens.harvard.edu
|
ARM 239
|